shelf life calculator for pharmaceutical products
Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology Using Industry Data. Type desired taa trt values.
This can also be used to determine the shelf life of a product prior to purchase.
. Shelf life calculation of drugs. This online service helps you to know how long your product is in good condition. Shelf life calculator for pharmaceutical products.
The International Conference on Harmonisation ICH of Technical Requirements for the Registration of Pharmaceuticals for Human Use guidance document Q1AR2 ICH Q1A defines shelf life as The time period during which a drug product is expected to remain within the approved shelf life specification provided that it is stored under the conditions defined on. Minimum data of three batches are used to estimate the. 1 the need to make statistical approaches more compatible with larger and larger amounts of data collected under.
This can also be used to determine the shelf life of a product prior to purchase. Pharmaceutical industry even after sitting on the shelf for a long time or when exposed to various. For the products stored at room temperature the assessment should be started from the occurrence of the significant change in product stored in accelerated conditions.
What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. Jan 05 the dates must be expressed numerically and chronologically day month year but the month can be expressed in letters. SHELF LIFE CALCULATION Shelf life is the period of time from the date of manufacture that a drug product is expected to remain within its approved product specification while stored under defined conditions.
In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field. Product name Shelf Life Start Date Todays Date Expiration Date. Also it is necessary to find the mark.
Best before 24 jan at least the month and the year for products with a shelf life over three months eg. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration date of a drug product and the stability testing needed to ensure that. ICH Q1E guideline provides guidance for the estimation of the shelf life of the pharmaceutical products and substances.
Pharmaceutical industry even after sitting on the shelf for a long time or when exposed to various. For estimating the shelf life of pharmaceutical products with stability data. Shelf life is determined by the evaluation of whole stability data of the product.
After entering data you push button Check and calculator will show you if. 24 months 36 months to a maximum of 60. Long time studies on pharmaceutical products are carried out over extended time periods till the formulation fails its specifications under the recommended storage conditions.
Statistica Sinica 112001 737-745 DRUG SHELF-LIFE ESTIMATION Jun Shao and Shein-Chung Chow University of Wisconsin and StatPlus Inc. Shelf life calculator in months. The shelf-life of a drug product is the time that the average drug charac- teristic eg potency remains within an approved specification after manufacture.
Typically storage is done at 250C - 20C and RH of 60 - 5 for up to 60 months. Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product. Enter all three dates to the left to see the remaining shelf life.
The interest in alternative methods was stimulated by several factors. Expiry with date in days in months or in years. Enter all three dates to the left to see the.
Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product. Shelf life is a product of physical microbiological and chemical processes triggered by any one of a multitude of contributing factors. Shelf life is typically expressed in units of months ie.
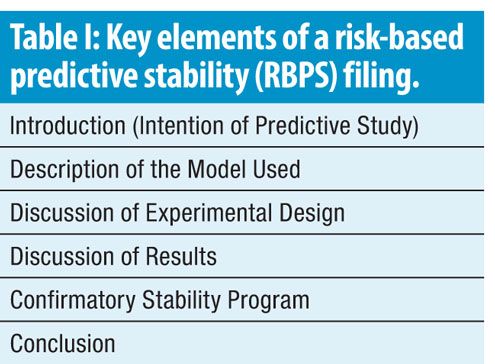
Risk Based Predictive Stability For Pharmaceutical Development A Proposed Regulatory Template

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know
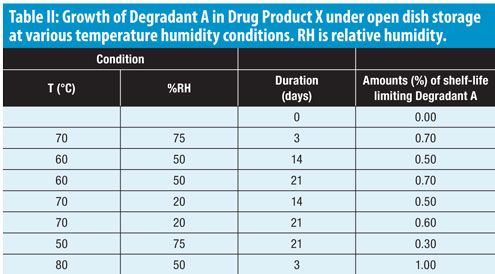
Risk Based Predictive Stability For Pharmaceutical Development A Proposed Regulatory Template

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know

Desiccant Faq Tropack Gmbh Trockenmittel

Pdf Assessing Shelf Life Using Real Time And Accelerated Stability Tests

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know
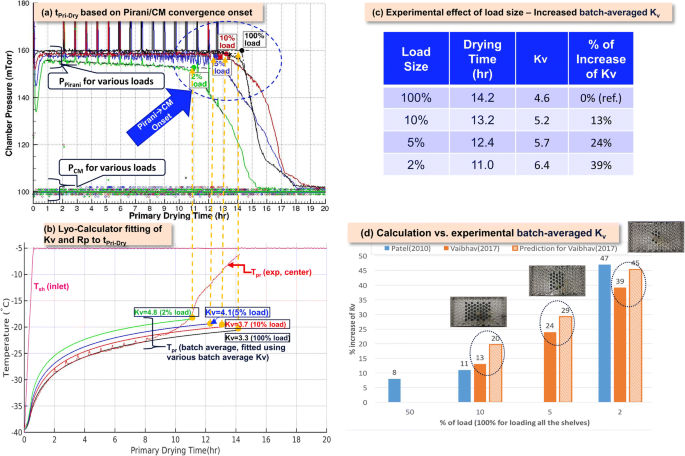
Recommended Best Practices For Lyophilization Validation 2021 Part I Process Design And Modeling Springerlink

Pdf Assessing Shelf Life Using Real Time And Accelerated Stability Tests

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know

Significance Of Shelf Life Studies On Pharmaceutical Products Lab Training Com

Risk Based Predictive Stability For Pharmaceutical Development A Proposed Regulatory Template